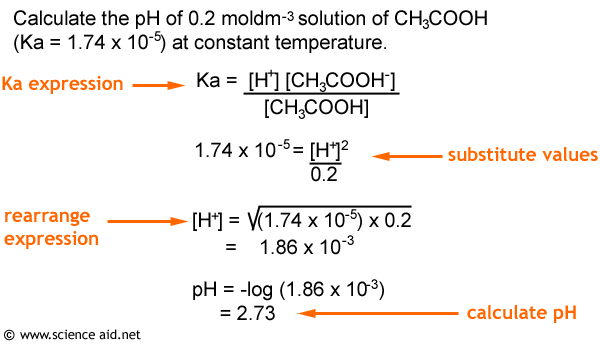
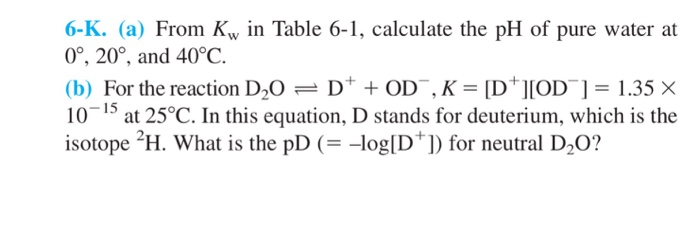
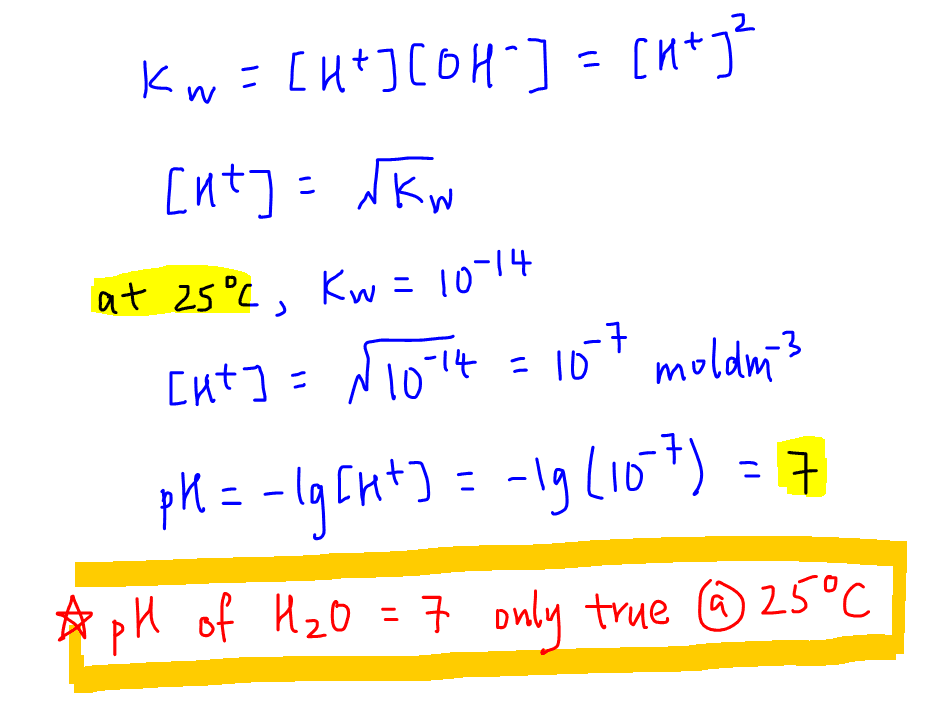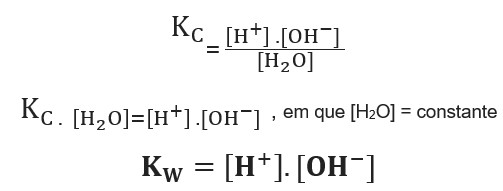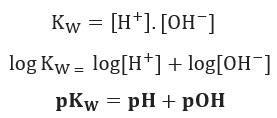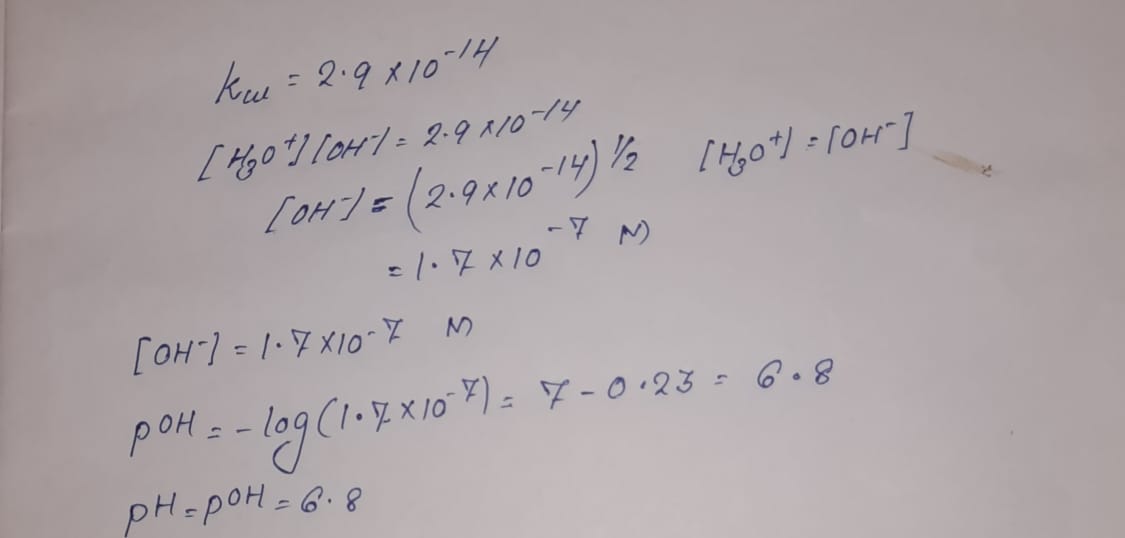PPT - Kw and Temperature Energy + H 2 O ⇋ H + + OH - @ 25 o C Kw = 1.0 x 10 -14 Higher Temperatures @ 50 o C Kw PowerPoint Presentation - ID:1080833
The ionization constant for water (Kw) is 9.311 × 10−14 at 60 °C. What is the [H3O+], [OH−], pH, and pOH for pure water at 60 °C? Thanks. - TopScience - Quora
![PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819 PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819](https://image2.slideserve.com/5054819/slide13-l.jpg)
PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819

pH from Base concentration and Ionic Product of Water calculation Workthrough - A2 Chemistry - YouTube

AutoIonization of Water, Ion Product Constant - Kw, Calculating H3O+, OH-, and pH Using Ice Tables - YouTube
![SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of](https://cdn.numerade.com/ask_previews/ed96ab60-48fb-4213-8fd0-3c129172f46d_large.jpg)
SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of
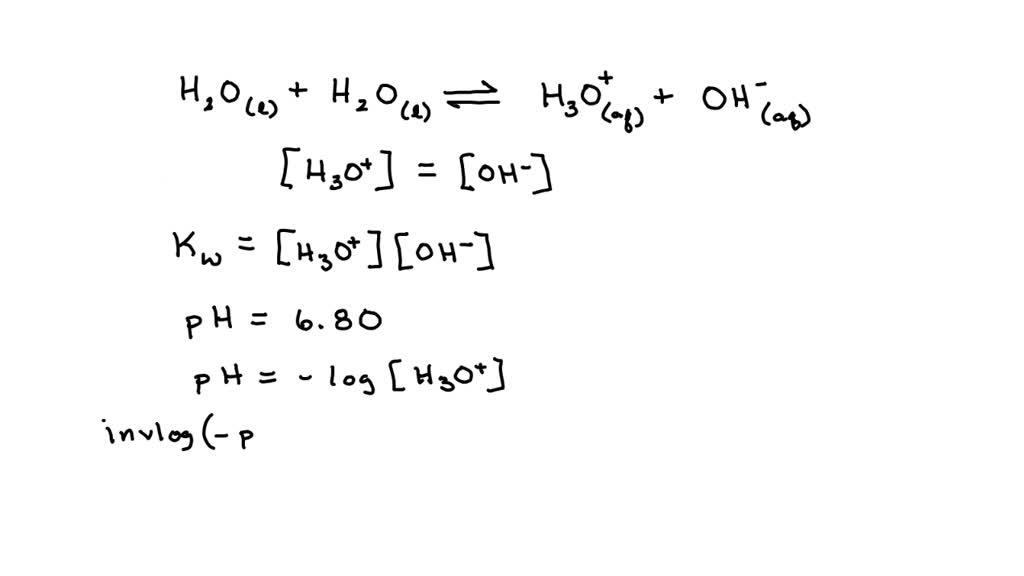
![Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy](https://cdn.kastatic.org/ka_thumbnails_cache/e67920d2-30a0-40ef-bcfb-a7761c9673f6_1280_720_base.png)